Driving Biotherapeutic R&D
BioGaia Pharma AB is a daughter company to BioGaia AB applying 25 years of probiotic experience to the research and development of new biotherapeutic product platforms.
Through an established network of research institutions and companies, BioGaia Pharma’s mission is to match early research on candidates from the microbiome with unmet medical needs to develop drug platforms.
BioGaia Pharma identifies opportunities and selects projects at discovery or pre-clinical stage with a well-defined medical need and will take viable therapeutic drug platforms through regulatory, product and clinical development phases. Platforms will be out-licensed at a suitable phase of development.
Microbial candidates will have the potential to prevent or treat disease either by influencing the host microbiome or through a direct communication with the body.
Product Pipeline
BioGaia Pharma is focused on two early phase programs. Approval has been secured for clinical studies in both programs and recruitment began 2022.
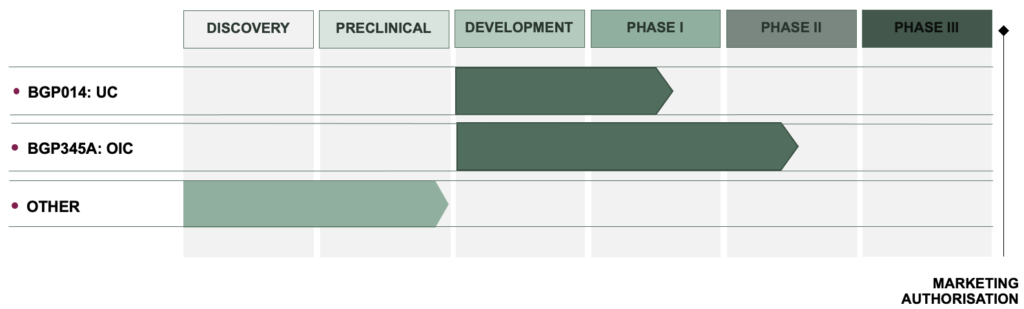
BGP014 is targeted for Ulcerative Colitis (UC). UC is a debilitating condition characterised by chronic inflammation in the intestinal tract affecting roughly 2,5 million patients in Europe and the US alone. There is no cure for UC and patients need life-long treatment prescribed according to the severity of the disease. The primary goal in UC therapy is to induce and maintain remission. First line standard of care is effective for a proportion of patients with few side effects but does not prevent regular flare ups and disease progression in >50% of patients necessitating step-up medication. The first step in this program is to look at safety, tolerability and preliminary efficacy of BGP014 in the approved Phase I multi-centre study with mild to moderate UC patients that will take place in Sweden.
For more information on the study: https://www.clinicaltrialsregister.eu/ctr-search/trial/2019-004578-25/SE
Do you have UC, live in Sweden and are interested in participating in the study?
BGP345A is being developed for the treatment of constipation in patients receiving opioid therapy for prescribed pain treatment. Opioids are an essential option for chronic pain, primarily back pain, rheumatism or post-operative pain, where non-opioid multimodal drugs or physical therapy are not providing relief. Opioid use can lead to a number of side effects with Opioid-Induced Constipation (OIC) being the most common with a varying prevelance of 41-81%. Current first line therapy is the use of laxatives but at least 45% of patients are laxative refractory and continue to have motility issues. The approved study is a Phase II multi-centre study to assess the safety and preliminary efficacy of BGP345A and will take place in France.
For more information on the study: https://www.clinicaltrialsregister.eu/ctr-search/trial/2021-000239-29/FR



